A green synthesis technique for fabricating carbon-coated magnetic nanoparticles
artikel lama ternyata…thn 2006…mudah2an bermangpaat..especially 4me
—
A green synthesis technique for fabricating carbon-coated magnetic nanoparticles
(Nanowerk Spotlight) Encapsulating metal nanoparticles inside carbon shells is of considerable significance but fraught with high manufacturing cost due to high energy consumption and intensive use of hardware. This cost issue limits their practical applications. Researchers in China have developed a novel, simple, efficient, and economical synthesis technique for the fabrication of carbon-encapsulated nanostructures where the carbonization is conducted at a relatively low temperature of 160°C in water and no toxic reagents are added. This new technique is facile and versatile, and suitable for the coating of other transition metal with carbon.
Metallic nanoparticles such as Fe, Co, and Ni are useful in various application fields of magnetism, including magnetic data-storage, ferrofluids, and biotechnology. Compared to polymer and silica shells, carbon shells exhibited much higher stability in various chemical and physical environments such as acid or base media, as well as at high temperatures and pressures. Carbon-coated ferromagnetic particles (metal@C) have opened the way for application in ultrahigh-density magnetic recording media, since the outer carbon layers can protect metals in the core from oxidation, act as a solid lubricant, and reduce the magnetic coupling between individual particles.
A whole range of synthesis techniques have been developed for encapsulating magnetic metal nanoparticles in carbon: arc techniques, magnetron and ion-beam co-sputtering, high-temperature annealing of the mixtures of carbon-based materials and metal precursors, catalytic chemical vapor deposition, pyrolysis of organometallic compound, catalytic decomposition of methane, explosion, and spraying method.
Professor Xian-Wen Wei from the College of Chemistry and Materials Science at Anhui Normal University in PR China explains his group’s new technique to Nanowerk: “Compared to other methods for formation of magnetic metal@C structures the carbonization reaction here is conducted at a relatively low temperature (160°C) in water and no toxic reagents are added. This method can be used to prepare other magnetic metal@C core-shell nanostructures, such as cobalt@C, which have been achieved in our laboratory. The two-step procedure (firstly synthesis of magnetic metal nanoparticles and secondly coating them with carbon) makes it easy to control the size, morphology and crystal phase of coated metals.”
In the approach that the Chinese researchers took, FeNi nanoparticles were prepared by a solution phase chemical reduction first, then an aqueous glucose solution was mixed with a suitable amount of FeNi nanoparticles, and then they were hydrothermally treated at 160°C for 3.5 h, leading to the formation of FeNi@C nanostructures.
Wei describes the three advantages of their two-step method:
- The two-step procedure makes it is easy to control the size, morphology and crystal phase of coated metals.
- The strategy is facile and versatile, and suitable for the coating of other transition metals with carbon.
- The carbonization reaction takes place at a relatively low temperature (160°C) in water and no toxic reagents are added.
The FeNi@C nanostructures with FeNi nanoparticles inside and a functionalized carbon surface outside may not only provide the opportunity to tailor the magnetic properties for magnetic storage devices and therapeutics but also make possible the loading of other functional molecules (e.g. enzymes, antigens) for clinic diagnostics, molecular biology, bioengineering, and catalysis.
Wei and his colleagues published their findings, titled “A solution phase fabrication of magnetic nanoparticles encapsulated in carbon” in the August 8, 2006 online issue of Nanotechnology.
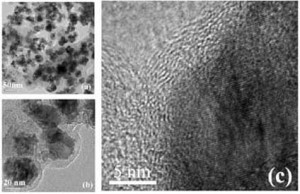
HR-TEM images revealed that all of the FeNi nanoparticles have a carbon shell (a), (b) after being hydrothermally treated with glucose for 3.5 h. The carbon shells tightly surrounded the core nanoparticles; no obvious voids can be observed between the core and the shell (a). This morphology is uniform throughout the sample. Most of the FeNi cores with a diameter of 35 nm shown by TEM are composed of several FeNi crystalloids with a size of about 10 nm, which accords with the FeNi crystalloid size calculated from XRD. The lattice fringe spacing of the core is about 0.21 nm (c). (Reprinted with permission from Institute of Physics Publishing)

